Introducing

A multiple trace element injection for neonatal and pediatric patients weighing less than 10 kg
Multrys™ (trace elements injection 4*, USP) is a combination of trace elements (zinc sulfate, cupric sulfate, manganese sulfate, and selenious acid) indicated in pediatric patients weighing less than 10 kg.
The concentration of each element in Multrys has been formulated to meet the needs of pediatric patients weighing less than
10 kg. Multrys is used as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.1
Formulated to Meet Today's Guidelines†
Multrys has been specifically developed to more closely align with the American Society for Parenteral and Enteral Nutrition (ASPEN) recommendations for trace element supplementation.2
Proven Stability
Stability studies support that Multrys can be safely stored for up to 9 days when added to the parenteral nutrition admixture and refrigerated.1
Consistent Supply
Multrys is proudly manufactured in America with Active Pharmaceutical Ingredients (API) and components sourced in America—our supply chain is short and less complicated. As a result, American Regent is uniquely positioned to provide you with consistent supply to help ensure critical medications reach patients faster.
| Product | Multrys™ |
| Approval Status | FDA Approved |
| Availability Status | Available |
| Pack NDC | 0517-9302-25 |
| Trace Elements per mL | Zinc 1,000 mcg Copper 60 mcg Manganese 3 mcg Selenium 6 mcg |
| Vial Type | Single Dose Vial |
| Fill Volume | 1 mL |
| Preservative | Preservative Free |
| Specific Gravity | 1.004 (g/mL) |
| Cap Color | Aqua |
| Aluminum Content | No more than 1,500 mcg/L of Aluminum |
| Pack Size | 25 Vials |
| Storage | Store at 20°C to 25°C (68°F to 77°F) |
| Trace Element Stability in TPN | Up to 9 days when added to the PN mixture and refrigerated |
 |
|
American Regent |
†Formulated to more closely align with the 2019 ASPEN Dosing Recommendation
Please see the Important Safety Information for Multrys below in addition to the product's Full Prescribing Information.
For pediatric and adult patients weighing at least 10 kg, please see the Tralement® (trace elements injection 4*, USP) product details page or view the product information bulletin.
*Each mL of Tralement contains zinc 3 mg, copper 0.3 mg, manganese 55 mcg, and selenium 60 mcg.
Multrys™ (trace elements injection 4*, USP) closely aligns with the daily recommendations for parenteral trace elements set forth by ASPEN.2
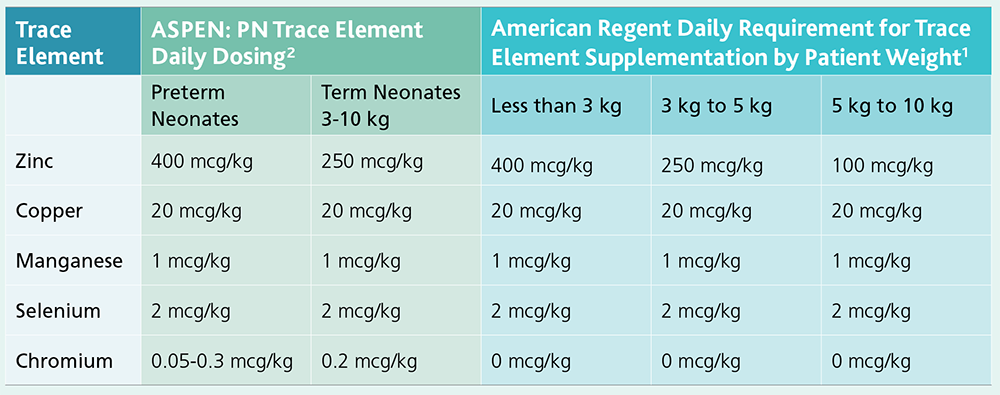
Multrys Dosing: Added to Parenteral Nutrition1
Pediatric patients weighing 0.4 kg to 0.59 kg:
The total recommended dosage of Multrys is 0.2 mL every other day. Daily supplementation of Zinc, Copper, and Selenium will be needed to meet daily requirements.
Pediatric patients weighing 0.6 kg to less than 10 kg:
The recommended dosage of Multrys is 0.3 mL/kg/day rounded to the nearest 0.1 mL for up to a maximum of 1 mL per day. The recommended volume of Multrys to be added to parenteral nutrition ranges from 0.2 mL per day to 1 mL per day based on body weight.
Additional Trace Element Supplementation with Multrys:
Multrys is recommended only for pediatric patients who require supplementation with all four of the individual trace elements (i.e., zinc, copper, manganese and selenium). To determine the additional amount of supplementation that is needed, compare the calculated daily recommended dosage based on the body weight of the patient to the amount of each trace element provided by Multrys and enteral nutrition sources.
Multrys is not recommended for pediatric patients who may require a lower dosage of one or more of these individual trace elements.
Avoid additional manganese supplementation with Multrys use. Accumulation of manganese in the brain can occur with long-term administration with higher than recommended dosage of 1 mcg/kg/day.
Always refer to the Full Prescribing Information for complete dosing.
1. Multrys (trace elements injection 4*) [package insert]. Shirley, NY: American Regent, Inc. 6/2021. 2. American Society for Parenteral and Enteral Nutrition (ASPEN) website: http://www.nutritioncare.org/uploadedFiles/Documents/Guidelines_and_Clinical_Resources/PN%20Dosing%201-Sheet-FINAL.pdf. Accessed December 4, 2020.
Contact Us
-
P: 800-645-1706
(8:00am–6pm Monday - Thursday and Friday 8:00am–4pm Eastern Time) -
P: 800-734-9236
F: 610-650-0170
E: pv@americanregent.comAdverse Drug Events (ADEs) may be reported to the FDA:
Phone: 1-800-FDA-1088
Web: www.fda.gov/medwatch -
P: 888-354-4855
(9am–5pm Eastern Time, Monday - Friday)
E: ma@americanregent.comFor drug information outside of normal business hours that cannot wait until the next day, please call:
P: 877-845-6371
-
American Regent, Inc.
5 Ramsey Road
Shirley, NY 11967P: 631-924-4000
F: 631-924-1731
MultrysTM (trace elements injection 4*, USP)
*Each mL of Multrys provides zinc 1,000 mcg, copper 60 mcg, manganese 3 mcg, and selenium 6 mcg.
For intravenous use
INDICATIONS AND USAGE
Multrys is a combination of trace elements (zinc sulfate, cupric sulfate, manganese sulfate, and selenious acid) indicated in neonatal and pediatric patients weighing less than 10 kg as a source of zinc, copper, manganese, and selenium for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.
IMPORTANT SAFETY INFORMATION
DOSAGE AND ADMINISTRATION
Important Administration Information
Multrys is supplied as a single-dose vial. Prior to administration, Multrys must be transferred to a separate parenteral nutrition container, diluted, and used as an admixture in parenteral nutrition solution.
Overview of Dosing
Prior to administration of parenteral nutrition solution containing Multrys, correct severe fluid, electrolyte and acid-base disorders. It is recommended only for patients who require supplementation with all four of the individual trace elements (zinc, copper, manganese and selenium). Multrys is not recommended for patients who may require a lower dosage of one or more of the individual trace elements. Avoid additional manganese supplementation with Multrys use.
CONTRAINDICATIONS
Contraindicated in patients with hypersensitivity to zinc or copper.
WARNINGS AND PRECAUTIONS
Pulmonary Embolism due to Pulmonary Vascular Precipitates: If signs of pulmonary distress occur, stop the parenteral nutrition infusion and initiate a medical evaluation.
Vein Damage and Thrombosis: Solution with an osmolarity of 900 mOsmol/L or greater must be infused through a central catheter.
Neurologic Toxicity with Manganese: Monitor for clinical signs and symptoms of neurotoxicity, whole blood manganese concentrations, and liver function tests. Discontinue Multrys and consider brain magnetic resonance imaging (MRI) if toxicity is suspected. Monitor patients for cholestasis or other biliary liver disease.
Hepatic Accumulation of Copper and Manganese: Assess for development of hepatic accumulation. Monitor concentrations of copper and manganese in patients with cholestasis or cirrhosis.
Aluminum Toxicity: Multrys contains aluminum that may be toxic. Patients with renal impairment and preterm infants, including preterm neonates, are particularly at risk.
Monitoring and Laboratory Tests: Monitor blood zinc, copper and selenium serum concentrations, whole blood manganese concentration, fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count, and coagulation parameters.
Hypersensitivity Reactions with Zinc and Copper: If hypersensitivity reactions occur, discontinue and initiate appropriate medical treatment.
ADVERSE REACTIONS
The following adverse reactions were identified in clinical studies or post-marketing reports:
- Neurologic toxicity with manganese
- Hepatic accumulation of copper and manganese
- Hypersensitivity reactions with zinc and copper
OVERDOSAGE
There are reports on overdosage in the literature for the individual trace elements.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236, or to the FDA by visiting www.fda.gov/medwatch or by calling 1-800-FDA-1088.