Email Notification
Receive Daily Availability Emails
Sign Up| Pack NDC# | Product | Strength | Supplied As | Shelf Pack | Inventory |
|---|---|---|---|---|---|
Pack NDC#
0517-6710-10

|
10% Calcium Chloride Injection, USP |
1 Gram/10 mL |
10 mL |
10 | In-Stock Shipping Weekly |
Pack NDC#
0517-7604-25
|
Acetylcysteine Solution, USP |
20% |
4 mL vial |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-7504-25

|
Acetylcysteine Solution, USP |
10% |
4 mL vial |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-9120-25

|
Aminocaproic Acid Injection, USP |
250 mg/mL |
20 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-9191-25
|
Aminocaproic Acid Injection, USP |
250 mg/mL |
20 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-1004-25
|
Atropine Sulfate Injection, USP |
0.4 mg/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-1001-25
|
Atropine Sulfate Injection, USP |
1 mg/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0720-01

|
Betamethasone Sodium Phosphate and Betamethasone Acetate Injectable Suspension, USP |
6 mg/mL |
5 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0791-01
|
Betamethasone Sodium Phosphate and Betamethasone Acetate Injectable Suspension, USP |
6 mg/mL |
5 mL |
1 | Out of Stock |
Pack NDC#
0517-0799-01
|
Betamethasone Sodium Phosphate and Betamethasone Acetate Injectable Suspension, USP |
6 mg/mL |
5 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-2502-10

|
Caffeine and Sodium Benzoate Injection, USP† †Drug is Not FDA-Approved |
250 mg/mL |
2 mL |
10 | In-Stock Shipping Weekly |
Pack NDC#
0517-1820-01
|
Chlorothiazide Sodium For Injection, USP |
500 mg |
Single-dose vial |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0031-25
|
Cyanocobalamin (B12) Injection, USP |
1000 mcg/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0130-01

|
Cyanocobalamin (B12) Injection, USP |
1000 mcg/mL |
30 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0032-25
|
Cyanocobalamin (B12) Injection, USP |
1000 mcg/mL |
10 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-9702-25
|
Droperidol Injection, USP |
5 mg/2 mL |
2 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-1980-05

|
Dicyclomine Hydrochloride Injection, USP |
20 mg/2 mL |
2 mL |
5 | In-Stock Shipping Weekly |
Pack NDC#
0517-3030-01
|
Epinephrine Injection, USP |
30 mg/30 mL (1 mg/mL) |
30 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0420-01
|
Estradiol Valerate Injection, USP |
20 mg/mL |
5 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0440-01
|
Estradiol Valerate Injection, USP |
40 mg/mL |
5 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0710-01
|
Fomepizole Injection |
1 g/mL |
1.5 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-4605-25

|
Glycopyrrolate Injection, USP |
0.2 mg/mL |
5 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-4620-25

|
Glycopyrrolate Injection, USP |
0.2 mg/mL |
20 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-4601-25
|
Glycopyrrolate Injection, USP |
0.2 mg/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-4602-25

|
Glycopyrrolate Injection, USP |
0.2 mg/mL |
2 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0901-25

|
HydrALAZINE Hydrochloride Injection, USP |
20 mg/mL |
1 mL Single Dose Vial |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-4201-25

|
Hydroxyzine HCl Injection, USP |
25 mg/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-5602-25
|
Hydroxyzine HCl Injection, USP |
50 mg/mL |
2 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-5601-25

|
Hydroxyzine HCl Injection, USP |
50 mg/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0650-01
|
Injectafer® (ferric carboxymaltose injection) |
750 mg iron/ |
15 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0602-01
|
Injectafer® (ferric carboxymaltose injection) |
100 mg iron/ |
2 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-1045-05
|
Levocarnitine Injection, USP |
1 gram/5 mL |
5 mL |
5 | In-Stock Shipping Weekly |
Pack NDC#
0517-1075-01
|
Levocarnitine Injection, USP |
4 grams/20 mL |
20 mL |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-0740-20
|
Methylergonovine Maleate Injection, USP |
0.2 mg/mL |
1 mL |
20 | In-Stock Shipping Weekly |
Pack NDC#
0517-9302-25
|
Multrys® (trace elements injection 4*, USP)
*Each mL contains zinc 1,000 mcg, copper 60 mcg, |
* |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0735-10

|
niCARdipine Hydrochloride Injection, USP |
2.5 mg/mL |
10 mL |
10 | Out of Stock |
Pack NDC#
0517-4810-25
|
Nitroglycerin Injection, USP |
5 mg/mL |
10 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0955-01
|
Olanzapine For Injection |
10 mg/Vial |
Single Use Vial |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-4300-01
|
Paclitaxel Protein-Bound Particles for Injectable Suspension (Albumin bound) |
100 mg/vial |
Single Dose Vial |
1 | In-Stock Shipping Weekly |
Pack NDC#
0517-4002-25

|
Papaverine HCl Injection, USP† †Drug is Not FDA-Approved |
30 mg/mL |
2 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-2051-25

|
Potassium Phosphates Injection, USP |
Phosphorus 15 mmol/5 mL and Potassium 22 mEq/5 mL |
5 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-2102-25

|
Potassium Phosphates Injection, USP |
Phosphorus 45 mmol/15 mL and Potassium 66 mEq/15 mL |
15 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-2505-25

|
Potassium Phosphates Injection, USP |
Phosphorus 150 mmol/50 mL and Potassium 220 mEq/50 mL |
50 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-0374-05
|
ProvayBlue® (methylene blue) Injection, USP |
50 mg/10 mL |
10 mL |
5 | In-Stock Shipping Weekly |
Pack NDC#
0517-0381-05
|
ProvayBlue® (methylene blue) Injection, USP |
50 mg/10 mL |
10 mL |
5 | In-Stock Shipping Weekly |
Pack NDC#
0517-6560-05

|
Selenious Acid Injection, USP |
600 mcg/10 mL |
10 mL |
5 | In-Stock Shipping Weekly |
Pack NDC#
0517-6502-10

|
Selenious Acid Injection, USP |
12 mcg/2 mL |
2 mL |
10 | In-Stock Shipping Weekly |
Pack NDC#
0517-7305-25

|
Sodium Phosphates Injection, USP |
15 mM P/5 mL (3 mM P/mL) |
5 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-7315-25

|
Sodium Phosphates Injection, USP |
45 mM P/15 mL (3 mM P/mL) |
15 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-7350-25

|
Sodium Phosphates Injection, USP |
150 mM P/50 mL (3 mM P/mL) |
50 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-9305-25
|
Tralement® (trace elements injection 4*, USP)
*Each mL contains zinc 3 mg, copper 0.3 mg, |
* |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-1020-25
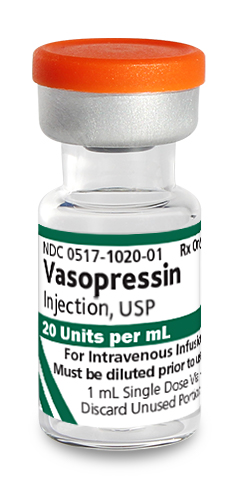
|
Vasopressin Injection, USP |
20 Units/mL |
1 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-1030-01
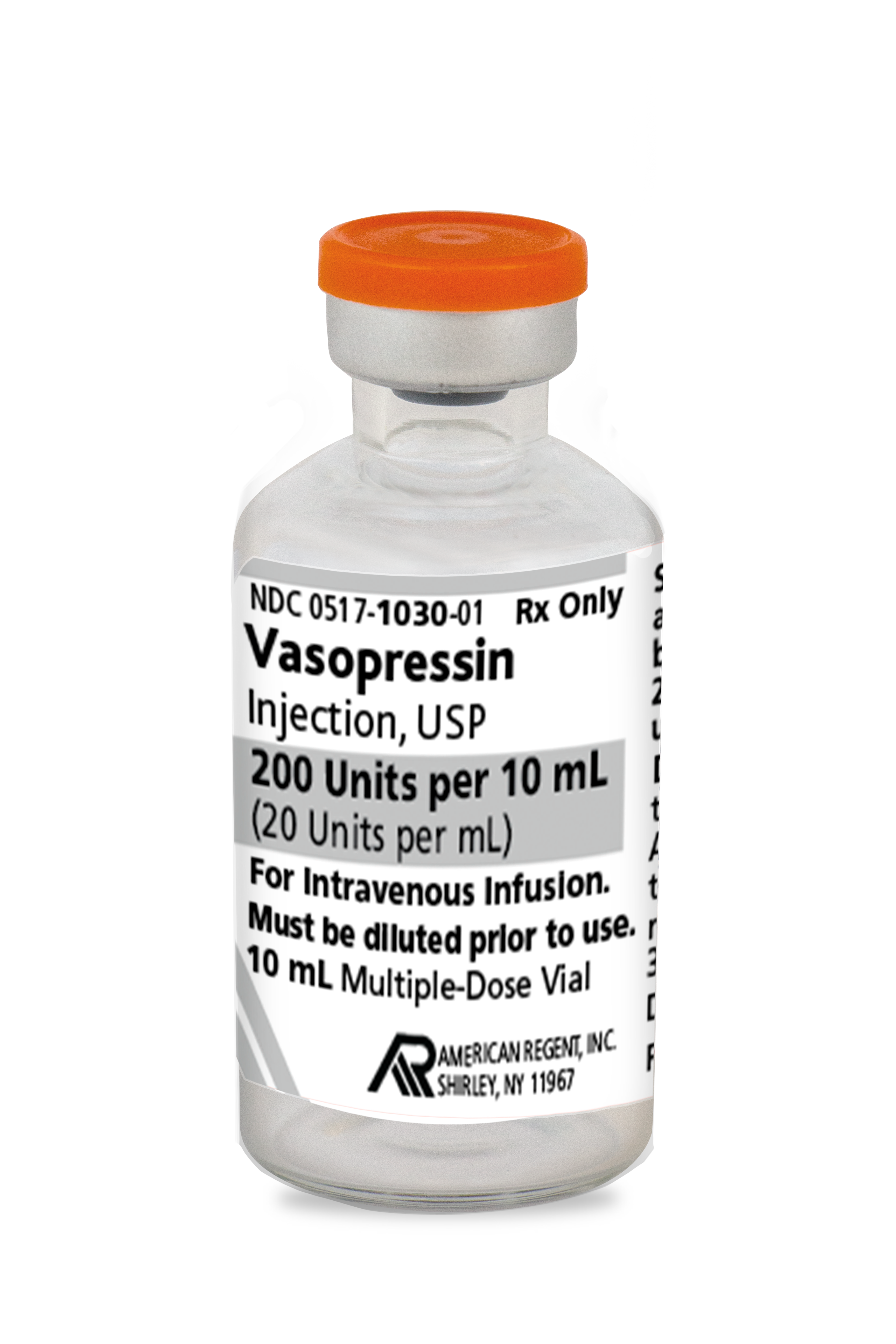
|
Vasopressin Injection, USP |
20 Units/mL |
10 mL |
1 | Available - Short Dated |
Pack NDC#
0517-2310-05
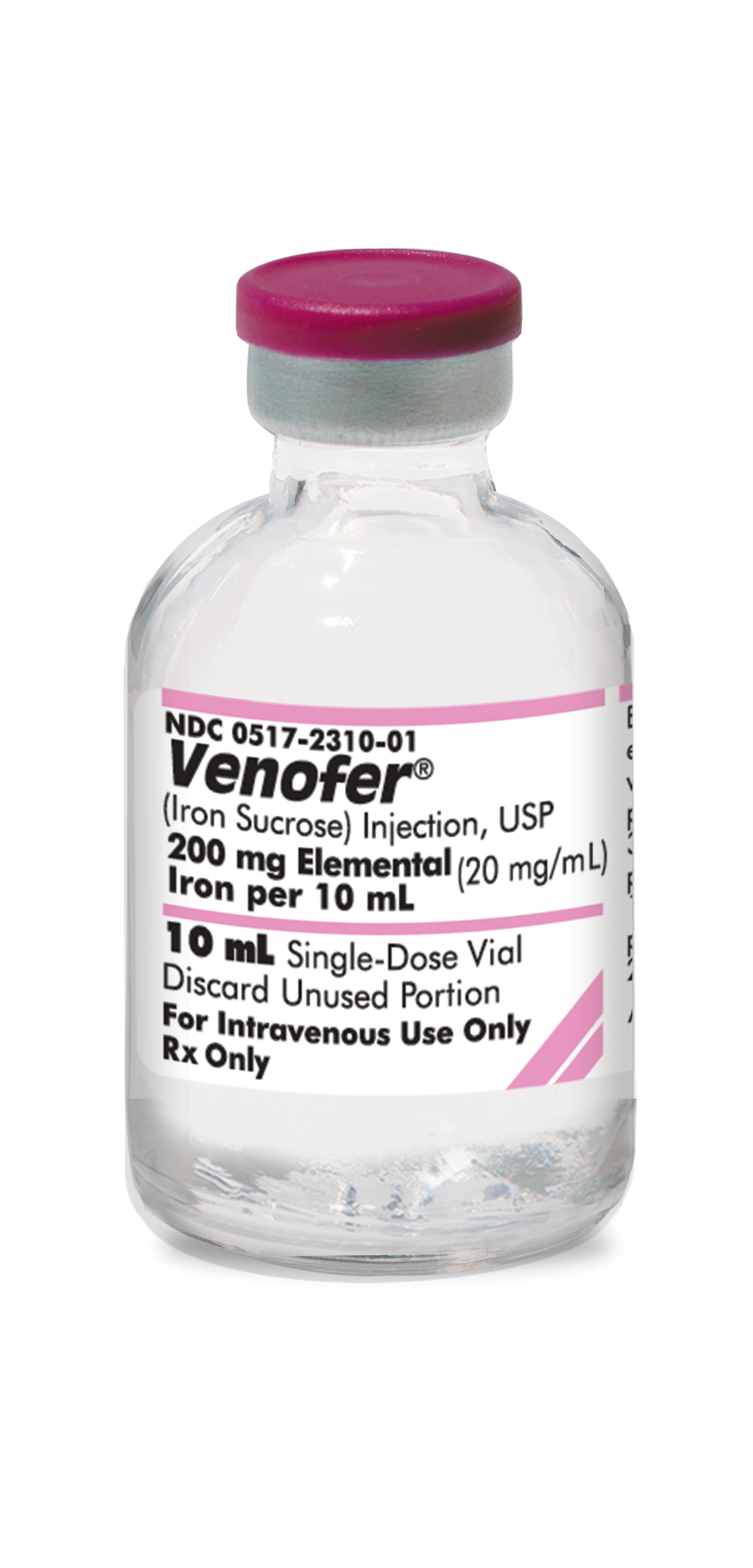
|
Venofer® iron sucrose injection, USP |
200 mg/10 mL |
10 mL |
5 | In-Stock Shipping Weekly |
Pack NDC#
0517-2325-10
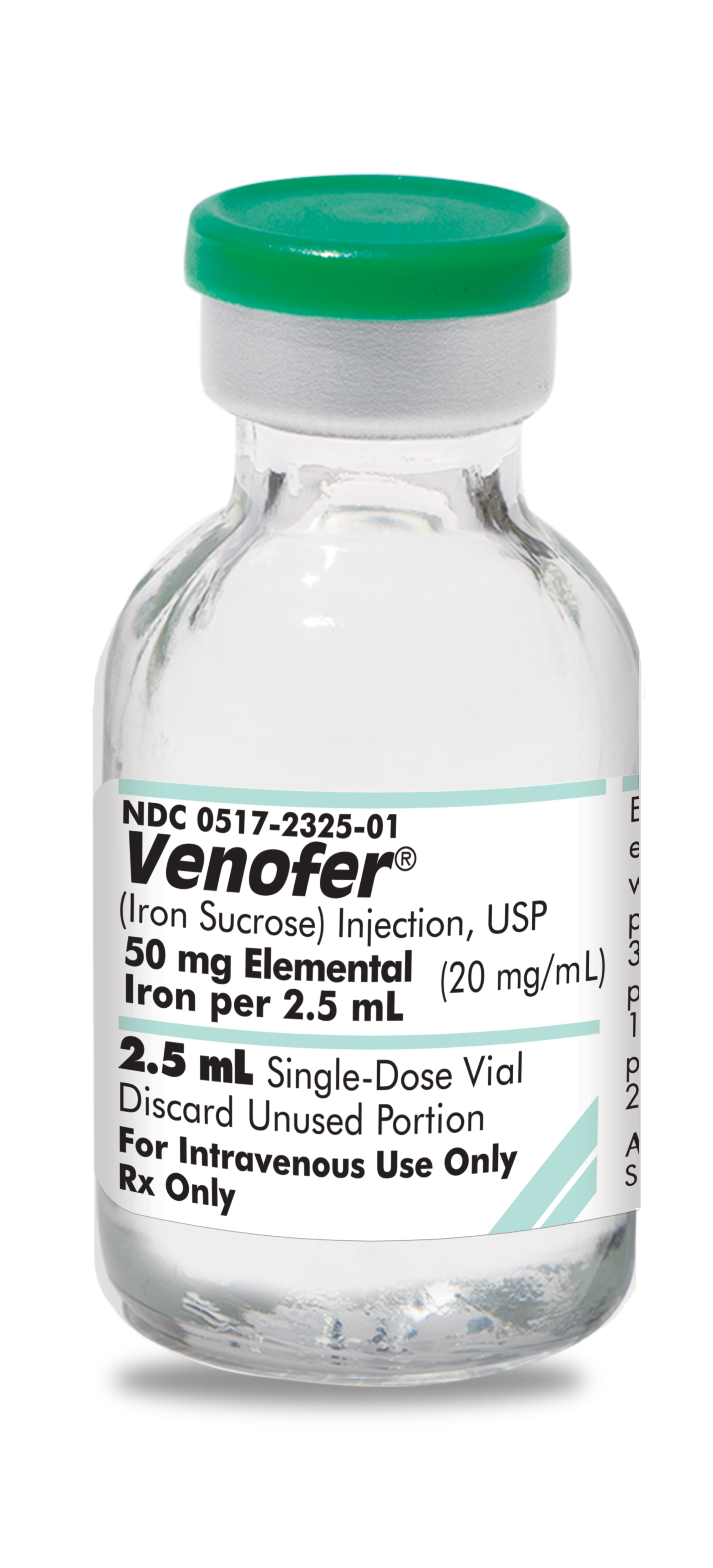
|
Venofer® iron sucrose injection, USP |
50 mg/2.5 mL |
2.5 mL |
10 | In-Stock Shipping Weekly |
Pack NDC#
0517-2340-10
|
Venofer® iron sucrose injection, USP |
100 mg/5 mL |
5 mL |
10 | In-Stock Shipping Weekly |
Pack NDC#
0517-2340-25

|
Venofer® iron sucrose injection, USP |
100 mg/5 mL |
5 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-6103-25
|
Zinc Sulfate Injection, USP |
3 mg/mL |
10 mL |
25 | Out of Stock |
Pack NDC#
0517-8005-25
|
Zinc Sulfate Injection, USP |
5 mg/mL |
5 mL |
25 | In-Stock Shipping Weekly |
Pack NDC#
0517-6101-25

|
Zinc Sulfate Injection, USP |
1 mg/mL |
10 mL |
25 | In-Stock Shipping Weekly |
FIND OUT FIRST
Receive daily availability emails
News Center
American Regent introduces Epinephrine Injection, USP in
30 mL; FDA-Approved and “AP” Rated
30 mL; FDA-Approved and “AP” Rated
Shirley, NY – October 15, 2024:
American Regent announces the launch and availability of 30 mL Epinephrine Injection, USP.
St. John’s University and American Regent Post-Doctoral Pharmaceutical Industry Fellowship Now Recruiting
Melville, NY – September 9, 2024:
Learn more about the St. John’s University and American Regent Post-Doctoral Pharmaceutical Industry Fellowship by downloading our brochure. Information on how to apply to our program as well as key deadlines are included.
American Regent Introduces Sodium Phosphates Injection, USP; FDA-Approved and “AP” Rated
Shirley, NY – July 2, 2024:
American Regent announces the launch and availability of Sodium Phosphates Injection, USP, which is FDA-approved and therapeutically equivalent to Sodium Phosphates.