Contact Us
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.
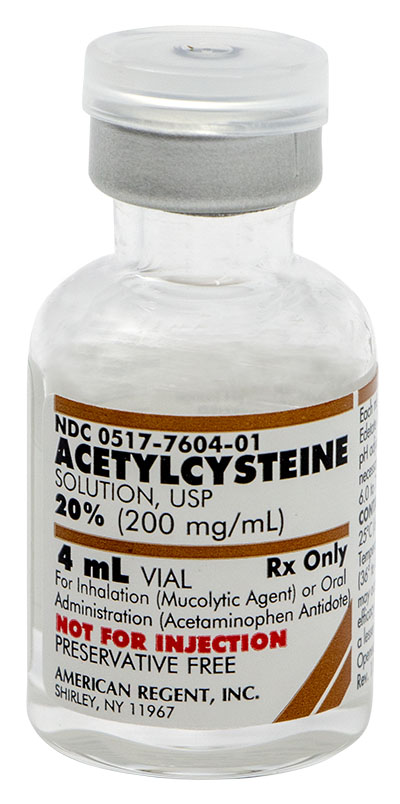
ACETYLCYSTEINE AS A MUCOLYTIC AGENT
CONTRAINDICATIONS
Acetylcysteine is contraindicated in those patients who are sensitive to it.
WARNINGS
After proper administration of acetylcysteine, an increased volume of liquefied bronchial secretions may occur. When cough is inadequate, the open airway must be maintained by mechanical suction if necessary. When there is a mechanical block due to foreign body or local accumulation, the airway should be cleared by endotracheal aspiration, with or without bronchoscopy. Asthmatics under treatment with acetylcysteine should be watched carefully. Most patients with bronchospasm are quickly relieved by the use of a bronchodilator given by nebulization. If bronchospasm progresses, the medication should be discontinued immediately.
PRECAUTIONS
General
With the administration of acetylcysteine, the patient may initially observe a slight disagreeable odor that is soon not noticeable. With a face mask, there may be stickiness on the face after nebulization. This is easily removed by washing with water.
Under certain conditions, a color change may occur in the opened bottle. The light purple color is the result of a chemical reaction, which does not significantly affect the safety or mucolytic effectiveness of acetylcysteine.
Continued nebulization of acetylcysteine solution with a dry gas will result in an increased concentration of the drug in the nebulizer because of evaporation of the solvent. Extreme concentration may impede nebulization and efficient delivery of the drug. Dilution of the nebulizing solution with appropriate amounts of Sterile Water for Injection, USP, as concentration occurs, will obviate this problem.
Pregnancy
Teratogenic Effects - Pregnancy Category B
This drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when acetylcysteine is administered to a nursing woman.
ADVERSE REACTIONS
Adverse effects have included stomatitis, nausea, vomiting, fever, rhinorrhea, drowsiness, clamminess, chest tightness, and bronchoconstriction. Clinically overt acetylcysteine-induced bronchospasm occurs infrequently and unpredictably, even in patients with asthmatic bronchitis or bronchitis complicating bronchial asthma. Acquired sensitization to acetylcysteine has been reported rarely. Sensitization has been confirmed after frequent and extended exposure to acetylcysteine. Reports of irritation to the tracheal and bronchial tracts have been received and, although hemoptysis has occurred in patients receiving acetylcysteine, such findings are not uncommon in patients with bronchopulmonary disease and a causal relationship has not been established.
Acetylcysteine should not be placed directly into the chamber of a heated (hot pot) nebulizer.
INDICATIONS AND USAGE
Acetylcysteine is indicated as adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions in such conditions as:
Chronic bronchopulmonary disease (chronic emphysema, emphysema with bronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis, and primary amyloidosis of the lung)
Acute bronchopulmonary disease (pneumonia, bronchitis, tracheobronchitis)
Pulmonary complications of cystic fibrosis
Tracheostomy care
Pulmonary complications associated with surgery
Use during anesthesia
Post-traumatic chest conditions
Atelectasis due to mucous obstruction
Diagnostic bronchial studies (bronchograms, bronchospirometry, and bronchial wedge catheterization).
ACETYLCYSTEINE AS AN ANTIDOTE FOR ACETAMINOPHEN OVERDOSE
CONTRAINDICATIONS
There are no contraindications to oral administration of acetylcysteine in the treatment of acetaminophen overdose.
WARNINGS
Generalized urticaria has been observed rarely in patients receiving oral acetylcysteine for acetaminophen overdose. If this occurs or other allergic symptoms appear, treatment with acetylcysteine should be discontinued unless it is deemed essential and the allergic symptoms can be otherwise controlled.
If encephalopathy due to hepatic failure becomes evident, acetylcysteine treatment should be discontinued to avoid further administration of nitrogenous substances. There are no data indicating that acetylcysteine influences hepatic failure, but this remains a theoretical possibility.
PRECAUTIONS
Occasionally, severe and persistent vomiting occurs as a symptom of acute acetaminophen overdose. Treatment with oral acetylcysteine may aggravate the vomiting. Patients at risk of gastric hemorrhage should be evaluated concerning the risk of upper gastrointestinal hemorrhage versus the risk of developing hepatic toxicity, and treatment with acetylcysteine given accordingly.
Dilution of the Acetylcysteine minimizes the propensity of oral acetylcysteine to aggravate vomiting.
ADVERSE REACTIONS
Oral administration of acetylcysteine, especially in the large doses needed to treat acetaminophen overdose, may result in nausea, vomiting, and other gastrointestinal symptoms. Rash with or without mild fever has been observed rarely.
INDICATIONS AND USAGE
Acetylcysteine, administered orally, is indicated as an antidote to prevent or lessen hepatic injury, which may occur following the ingestion of a potentially hepatotoxic quantity of acetaminophen. It is essential to initiate treatment as soon as possible after the overdose and, in any case, within 24 hours of ingestion.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report Adverse Drug Events to American Regent Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088
REF-0227 5/2014