Contact Us
By clicking Submit, you confirm that you accept our Privacy Policy and that you agree to your personal contact information being used to contact you and added to our database. If at any time you wish your personal information to be removed from the American Regent® database, please submit a message request to corpcommunications@americanregent.com.
CONTRAINDICATIONS
Hydroxyzine hydrochloride intramuscular solution is intended only for intramuscular administration and should not, under any circumstances, be injected subcutaneously, intra-arterially, or intravenously.
Hydroxyzine is contraindicated in patients with a prolonged QT interval.
This drug is contraindicated for patients who have shown a previous hypersensitivity to it.
Hydroxyzine, when administered to the pregnant mouse, rat, and rabbit, induced fetal abnormalities in the rat at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy.
WARNINGS
Tissue damage: Intramuscular hydroxyzine hydrochloride may result in severe injection site reactions (including extensive tissue damage, necrosis, and gangrene) requiring surgical intervention (including debridement, skin grafting, and amputation).
PRECAUTIONS
THE POTENTIATING ACTION OF HYDROXYZINE MUST BE CONSIDERED WHEN THE DRUG IS USED IN CONJUNCTION WITH CENTRAL NERVOUS SYSTEM DEPRESSANTS SUCH AS NARCOTICS, BARBITURATES, AND ALCOHOL. Rarely, cardiac arrests and death have been reported in association with the combined use of hydroxyzine hydrochloride intramuscularly and other CNS depressants. Therefore, when central nervous system depressants are administered concomitantly with hydroxyzine, their dosage should be reduced up to 50 percent.
HYDROXYZINE MAY POTENTIATE NARCOTICS AND BARBITURATES, so their use in preanesthetic adjunctive therapy should be modified on an individual basis. When hydroxyzine is used preoperatively or prepartum, narcotic requirements may be reduced as much as 50 percent. Meperidine should be used with great caution and in reduced dosage in patients who are receiving other pre- and/or postoperative medications and in whom there is a risk of respiratory depression, hypotension, and profound sedation or coma occurring. Before using any medications concomitant with hydroxyzine, the manufacturer's Prescribing Information should be read carefully.
Since drowsiness may occur with the use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery while taking this drug.
CASES OF QT PROLONGATION AND TORSADE DE POINTES HAVE BEEN REPORTED DURING POSTMARKETING USE OF HYDROXYZINE. The majority of reports occurred in patients with other risk factors for QT prolongation or Torsade de Pointes (pre-existing heart disease, electrolyte imbalances, or concomitant arrhythmogenic drug use). Therefore, hydroxyzine should be used with caution in patients with risk factors for QT prolongation, congenital long QT syndrome, a family history of long QT syndrome, other conditions that predispose to QT prolongation, and ventricular arrhythmia, as well as recent myocardial infarction, uncompensated heart failure, and bradyarrhythmias. Caution is recommended during the concomitant use of drugs known to prolong the QT interval.
Acute Generalized Exanthematous Pustulosis (AGEP)
Hydroxyzine may rarely cause acute generalized exanthematous pustulosis (AGEP), a serious skin reaction characterized by fever and numerous small, superficial, nonfollicular, sterile pustules, arising within large areas of edematous erythema. Inform patients about the signs of AGEP. Discontinue hydroxyzine at the first appearance of a skin rash, worsening of pre-existing skin reactions which hydroxyzine may be used to treat, or any other sign of hypersensitivity. If signs or symptoms suggest AGEP, use of hydroxyzine should not be resumed and alternative therapy should be considered. Avoid cetirizine or levocetirizine in patients who have experienced AGEP or other hypersensitivity reactions with hydroxyzine, due to the risk of cross-sensitivity.
ADVERSE REACTIONS
Therapeutic doses of hydroxyzine seldom produce impairment of mental alertness. However, drowsiness may occur; if so, it is usually transitory and may disappear in a few days of continued therapy or upon reduction of the dose. Dryness of the mouth may be encountered at higher doses.
Involuntary motor activity, including rare instances of tremor and convulsions, has been reported, usually with doses considerably higher than those recommended. Hydroxyzine hydrochloride is associated with acute generalized exanthematous pustulosis (AGEP) in postmarketing reports.
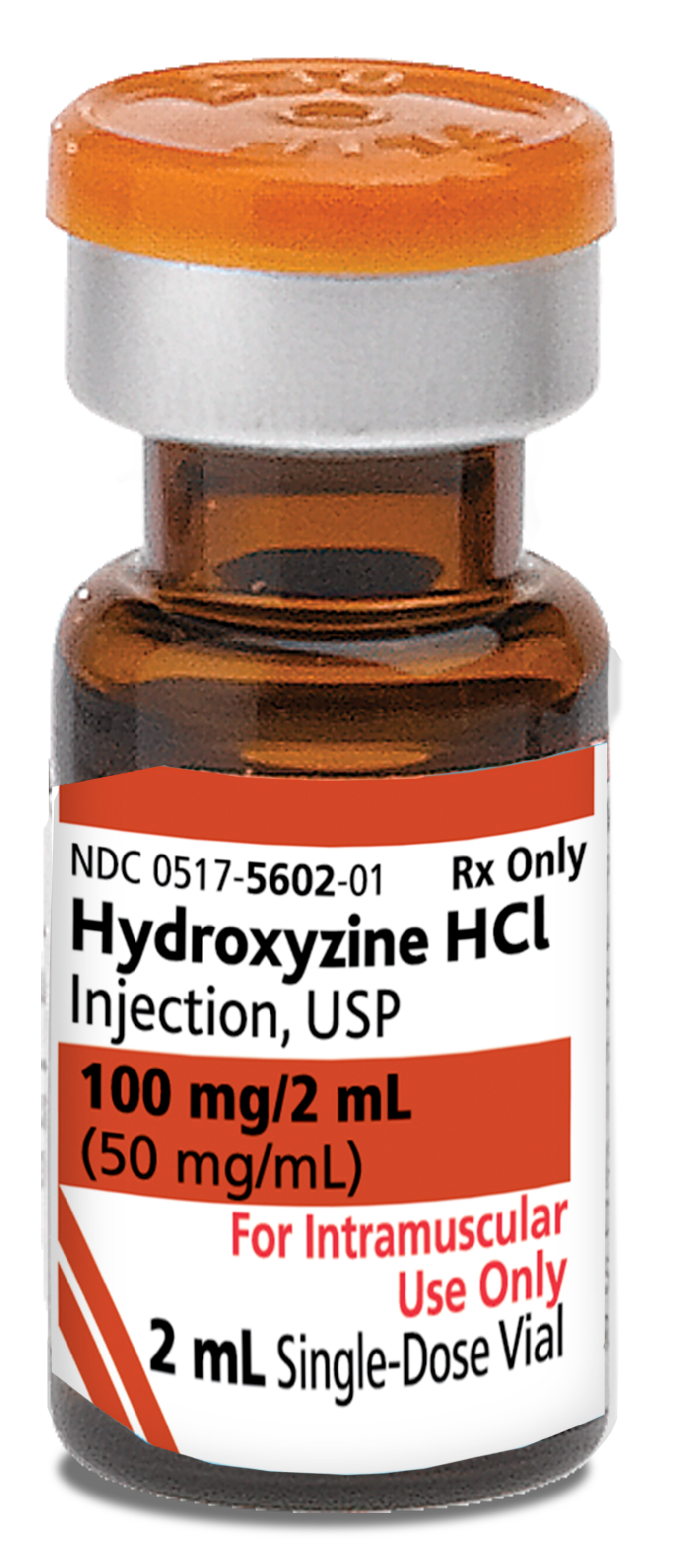
INDICATIONS AND USAGE
Hydroxyzine hydrochloride intramuscular solution is useful in treating the following types of patients when intramuscular administration is indicated:
1. The acutely disturbed or hysterical patient.
2. The acute or chronic alcoholic with anxiety withdrawal symptoms or delirium tremens.
3. As pre- and postoperative and pre- and postpartum adjunctive medication to permit reduction in narcotic dosage, allay anxiety, and control emesis.
For additional safety information, please see Full Prescribing Information.
You are encouraged to report Adverse Drug Events to American Regent, Inc. at 1-800-734-9236 or to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
REF-0516 10/2016